A meeting conducted by the CDC’s vaccine advisory committee, reshaped by US Health and Human Services Secretary Robert F. Kennedy Jr., wrapped up with confusion and divided votes.
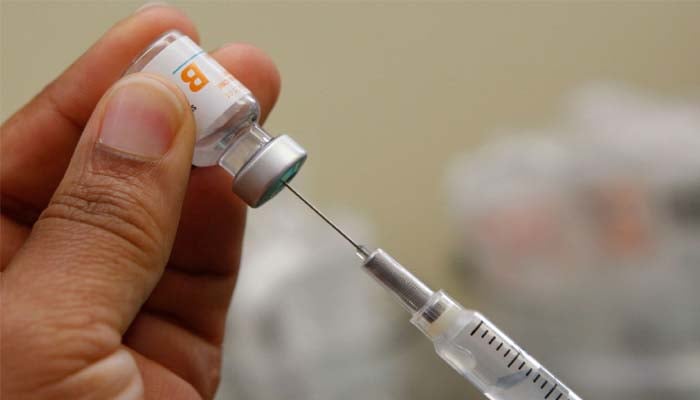
The committee postponed a vote on hepatitis B vaccines for newborns until Friday, September 19, 2025.
Since 1991, neonates have received vaccination at birth, minimising cases for 18,000 to nearly 20 annually.
Members debated delaying the initial dose to one month if mothers test negative, a move critics could leave babies unprotected when they are at an increased risk.
Notably, Hepatitis B is highly contagious and can transmit through tiny amounts of blood and household contact.
Additionally, the committee voted 8-3 against providing the combined measles, mumps, rubella, and varicella (MMRV) vaccine to children under 4, citing an increased seizure risk.
However, for children in the federal Vaccines for Children (VFC) program, parents can choose between the MMRV booster or two separate doses.
The meeting further underscored the feud since Kennedy replaced all 17 former members in June with his own appointees, prompting criticism from ex-CDC leaders.
They warned that Kennedy’s changes could mislead and cause disruption in vaccine policy.
The meeting continued on Friday with votes on COVID-19 shots and hepatitis B. Critics stated that ACIP’s current moves risk confusing doctors and parents and shattering confidence during the early stage of life in childhood immunisation programs.
