The FDA has approved the game-changing first flu vaccine spray that can be self-administered!
On Friday, September 20, the United States Food and Drug Administration gave green light to AstraZeneca’s latest flu vaccine that can be used by the patient himself.
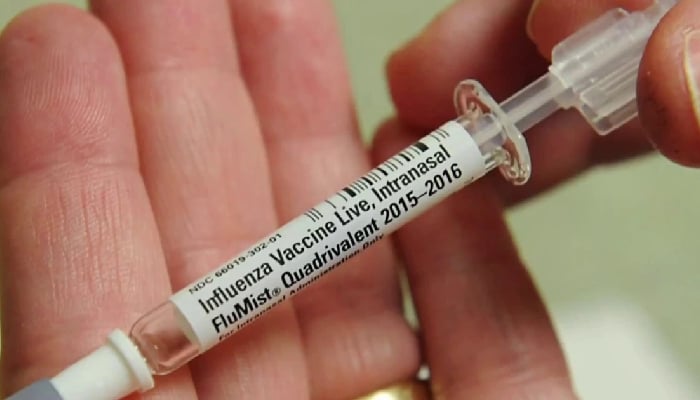
The vaccine, FluMist, is administered as a spray rather than the traditional injections that were injected with the help of a healthcare provider, making it the first of its kind.
AstraZeneca is gearing up to launch the vaccine through a third-party online pharmacy where the buyers would be required to undergo a complete screening and assessment to get their hands on the vaccine.
Flu is a highly contagious respiratory illness caused by the influenza virus, often prevalent in the US during the fall and winter seasons.
The director of the FDA’s Center for Biologics Evaluation and Research, Peter Marks, noted in his statement that “Today’s approval of the first influenza vaccine for self- or caregiver-administration provides a new option for receiving a safe and effective seasonal influenza vaccine, potentially with greater convenience, flexibility, and accessibility for individuals and families.”
FluMist, which was first manufactured by MedImmune before being acquired by AstraZeneca in 2007, was first approved by the FDA in 2003 with the patients’ ages ranging from 5 to 49. Its approval later expanded to children as young as two.