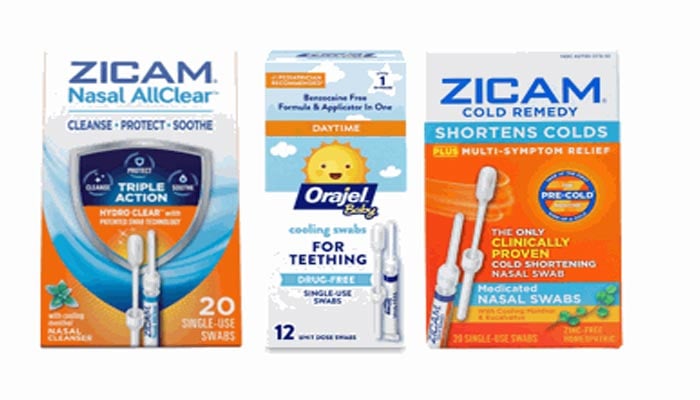
The U.S. Food and Drug Administration (FDA) announced the recall of some nasal and baby teething swabs from Zicam and Orajel all over the country because of higher contaminated fungus risk.
The announcement comes after the manufacturer, Church & Dwight Co., Inc., discovered the problem while testing, according to CBS News.
The issue was associated with the cotton components with fungi in the products, the company said.
Here are the recalled products:
- All lots of Zicam® Cold Remedy Nasal Swabs (UPC 732216301205)
- All lots of Zicam® Nasal AllClear Swabs (UPC 732216301656)
- All lots of Orajel™ Baby Teething Swabs (UPC 310310400002)
The FDA further stated that other products of Zicam and Orajel are not included as part of the recall and people can use it without worrying about contamination.
What to do if you have already bought recalled swabs?
If you have already purchased the recalled products, then you should immediately stop it, the FDA recommended.
Fungi-contaminated swabs may cause serious infections, especially in children, immunocompromised individuals, and individuals suffering from any other medical condition.
The FDA stated that the contamination may lead to fatal blood infections in rare cases.
So far, no illnesses have been reported, CBS News confirmed.








