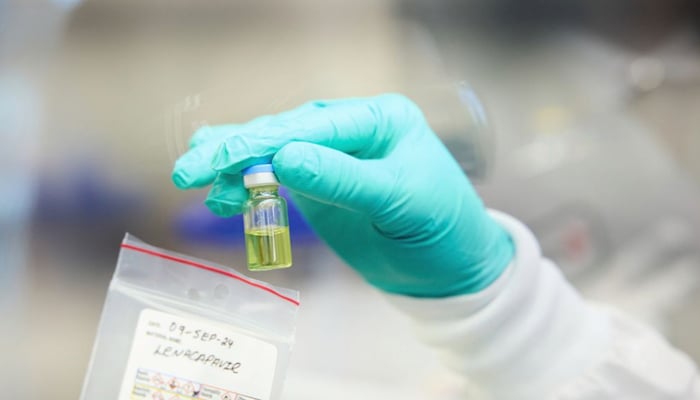
The Food and Drug Administration (FDA) approved a new vaccine to prevent HIV infection, lenacapavir, a long-acting injection, which is only required to be taken twice a year.
The drug showed nearly 100% effectiveness in clinical trials.
According to The New York Times, lenacapavir is manufactured by Gilead Sciences and sold under the name Yeztugo.
Mitchell Warren, head of the international HIV prevention group AVAC, stated, “We’re on the precipice of now being able to deliver the greatest prevention option we’ve had in 44 years of this epidemic.”
Lenacapavir is the second long-acting drug approved to prevent HIV.
For the study, the first, Apretude, is given every two months and it is used by about 21,000 Americans. In contrast, up to 500,000 individuals take daily pills to prevent HIV.
Health experts stated that a twice-yearly shot could transform HIV prevention, particularly for individuals who find it difficult to take pills every day.
For clinical trials, participants who received just two shots a year were protected from HIV, as reported by The Times.
Gilead has revealed the list price for its latest HIV prevention shot, Yeztugo (lenacapavir), which will be $28,218 per year; however, most people are not required to pay the complete amount due to insurance and Medicaid coverage.
In contrast, generic daily HIV prevention pills cost only $1 per dose, and Apretude, another long-term shot, is available for about $24,000 annually.
Advocates issued warnings regarding the high costs and insurance barriers that may limit access across the US.
Globally, 1.3 million people were newly infected with HIV in 2023, and over 39,000 infections occurred in the US, each costing an estimated $1.1 million in lifetime medical expenses.
For global distribution, Gilead will offer lenacapavir at no profit for up to two million individuals in low-income countries until local manufacturing builds up within two to three years.
Experts highlighted the significance of prevention, as it must remain a priority to avoid HIV.