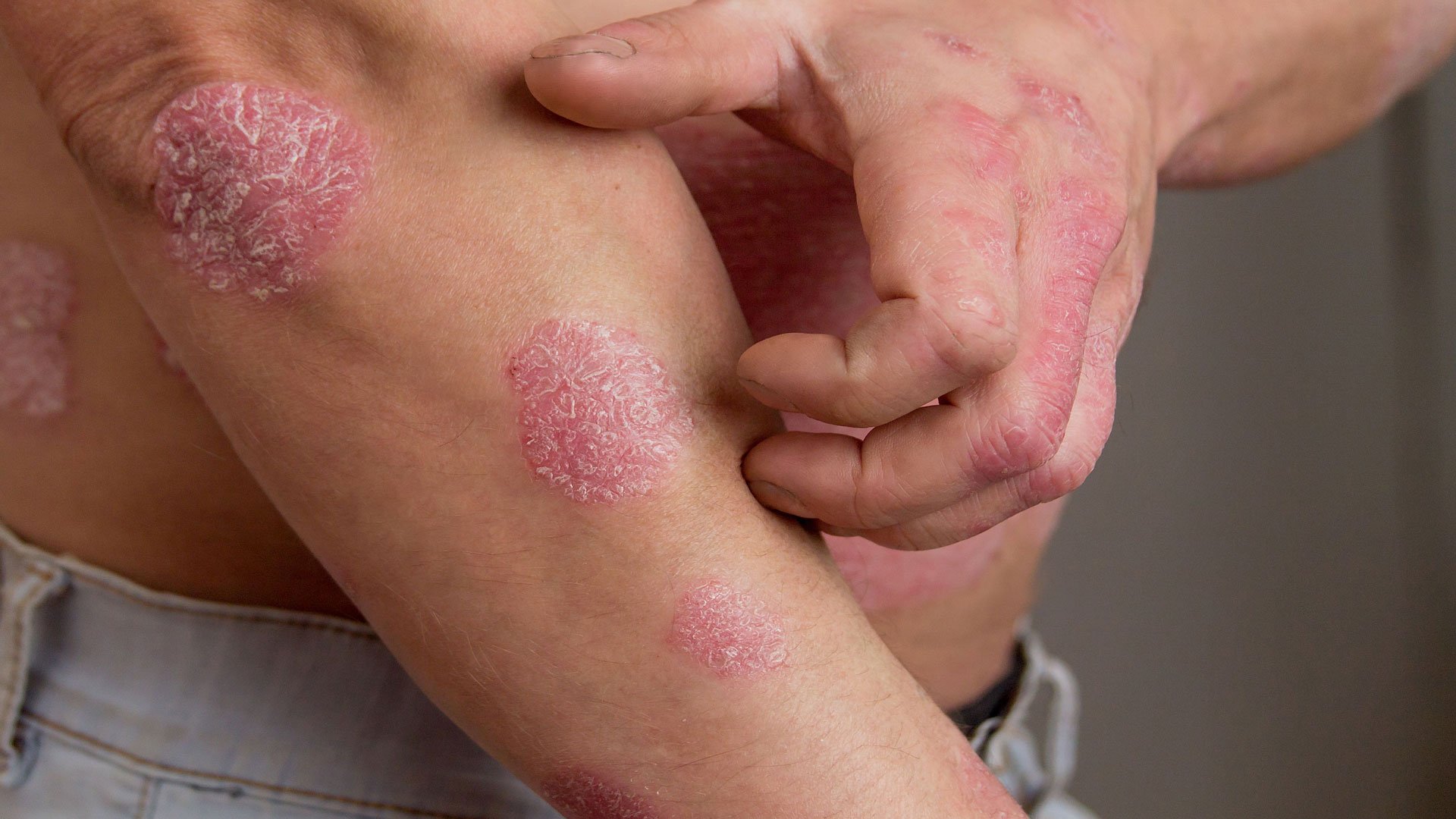
Alumis, a clinical-stage biopharmaceutical company, has announced two phase 3 trials of its TYK2 inhibitor envudeucitinib met their co-primary endpoints in adults with moderate to severe plaque psoriasis, making the medicine as a potential rival to Bristol Myers Squibb’s Sotyktu.
After receiving the news, Alumis’ shares spiked to double in premarket trading.
Nearly 1,700 patents were enrolled in those trials, with half getting envudeucitinib twice daily and the rest getting either Amgen’s Otezla (apremilast) or placebo.
At Week 16, 74% of patients treated with envudeucitinib successfully accomplished about 75% enhancement on the Psoriasis Area and Severity Index (PASI 75), while 59% sign reached a score of zero or one on the static Physician’s Global Assessment (sPGA), meeting both co-primary endpoints.
Alumis further reported that early separation from placebo by Week 4 and improved results, with reduced itching and feeling of discomfort.
While cross-trial comparisons should be interpreted cautiously, the results were better than those reported for Sotyktu that showed lower PASI 75, PASI 90, and PASI 100 response rates in its phase 3 trials.
Alumis plans to file for FDA approval in the second half of 2026, intensifying competitive pressure on Sotyktu, which has adversely impacted initial sales expectations. The company is also advancing envudeucitinib into lupus, with phase 2b data expected in the near future.