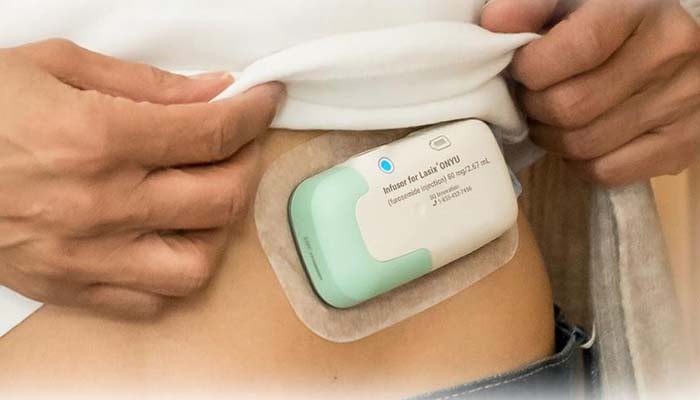
The FDA has granted approval to Lasix ONYU (furosemide injection), a new at-home variant of the widely used cardiac failure drug Lasix, developed by SQ Innovation, Inc.
The treatment enables specific patients to get subcutaneous infusions (under the skin) at home rather than in hospitals.
CEO of SQ Innovation, Dr. Pieter Muntendam stated, “Lasix ONYU could transform care for patients with worsening heart failure due to fluid overload.”
Pieter further mentioned plans to launch the therapy later this year.
Over 6.7 million Americans are suffering from heart failure—a number likely to reach 8.7 million by 2030.
Notably, nearly 1.2 million people get admitted to the hospitals annually.
The latest system delivers the similar drugs as conventional IV Lasix via a small, wearable device with a single-use component, minimising costs.
Clinical trials revealed 112% bioavailability in contrast to IV Lasix, with similar urine output and sodium loss, indicating comparable effectiveness.
Experts called the innovation a great step forward in cardiac failure management, allowing many patients to avoid prolonged hospitalisations.
Lasic ONYU will be distributed nationwide this quarter via leading pharmaceutical partners and select pharmacies.












